
Symbiosis Pharma Services specialises in the production of injectable pharmaceuticals for clinical and low volume commercial supply. Our integrated biopharma services help get your drug product released quickly and efficiently.

Finding a suitable GMP aseptic fill-finish manufacturer with the right capabilities, experience and a proven track record of delivering projects in short timelines can be a challenge – especially when handling advanced therapeutics such as viral vectors or antibody drug conjugates.
Symbiosis has extensive experience in taking precious drug product through manufacturing, testing, label and packaging, storage, distribution and QP release. Our expert project management team offers a single point of contact throughout each project, ensuring that communication happens as clearly and as frequently as you need.
Symbiosis’ GMP compliant biologic production facility is fully licenced by the MHRA, so rest assured, you’re in safe hands.
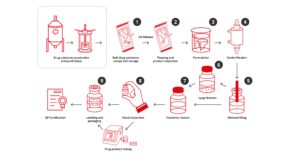

Establishing a production and purification process for your drug substance is a significant achievement. However, you still need to get this into a format that can be administered to the patient in a reliable and safe manner.
Consideration must be given to the final composition (e.g. buffers and any other additives) as well as any special handling and storage requirements for your product. The container system must also be considered as some therapies, especially advanced therapies, have limited compatibility with traditional glass vials.
Symbiosis has a range of validated options that can be used to successfully deliver your precious product to the patient quickly.
Learn more about the range of clinical biologics manufacturing and commercial services that we offer.

Symbiosis assists with a range of development services to get products from pre-clinical phases into clinical trials including formulation, lyophilisation cycle, and process development expertise.
Learn more

Symbiosis is fully licensed for the sterile filling of liquid and lyophilised investigational and commercial products. Aseptic manufacture is at the heart of what we do.
Learn more

We can integrate lyophilisation or gas overlay to liquid fill options as part of the aseptic filling process within our GMP facility.
Learn more

Our quality experts can certify clinical and commercial batches. Manufacturing to GMP regulations, we support QP certification for clinic or direct to you for onward processing.
Learn more

Symbiosis has dedicated suites and automation capability at our facility for the labelling and packaging of drug products for clinical trials.
Learn more

Our expert team can coordinate all your analytical and microbiological testing requirements. This allows you to keep your drug product in the hands of one reliable partner and accelerates delivery timelines.
Learn more