
Confidently meet the specific needs and tight timelines for your RNA or peptide sterile manufacturing with Symbiosis as your trusted partner.
Nucleic acid therapeutics can achieve long-lasting or even curative effects via gene inhibition, addition, replacement or editing. Breakthroughs in lipid formulation and GalNAc conjugation of modified oligonucleotides have paved the way to efficient delivery. Probably the most important breakthrough in the successful launch of two RNAi therapeutics concerns delivery using LNPs or conjugation chemistry for receptor-mediated uptake and tissue-specific targeting.
Symbiosis has experience of formulation and/or fill-finish of products of a number of these formulation approaches including protein/antibody conjugates, lipid conjugates, nanoparticles, and viral vectors.
Dedicated facility for development and cGMP manufacturing of your RNA and Peptide Therapies, including L-RNA Oligonucleotides, conjugated SiRNA, GalNAc modified RNA Oligonucleotides and SiRNA oligonucleotides. We are experts in small-scale commercial RNA/Oligonucleotide manufacturing.
>150 mL ≤50L
≤3000 vial

“Peptide drug development has made great progress in the last decade due to innovative technology being incorporated into production, modification, and analysis. There is significant demand to design proteins and, enzymes with drug delivery systems to target or mitigate disease or disorders. Over the last few years, Symbiosis have worked with several biotech and pharma companies who have been evolving exciting and novel RNA and peptide-based therapeutics, which have required lateral manufacturing and process design to optimise production. It is this experience that makes Symbiosis a standout aseptic fill finish CMO in this field”
Lorraine Dillon - Head of Technical, Symbiosis
 Find out out about our RNA and Oligonucleotide manufacturing capabilities and experience by downloading this information sheet.
Find out out about our RNA and Oligonucleotide manufacturing capabilities and experience by downloading this information sheet.
Symbiosis are the leading fast access CMO for clinical or small scale commercial manufacture and our offering is built upon the following principles
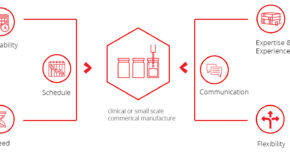
Waiting 6 or even 12 months for a filling slot can be detrimental. We work to your schedule and strive to have availability in as little as 8 weeks.
Delivering your product to the clinic on time is essential, given patients are often waiting. Therefore, we execute over 90% of projects within the agreed timeframe.
We understand the need for results is a critical factor in the progression of clinical trials. Our typical fill-finish campaign takes 12 weeks.
Our dedicated team has handled >600 filling campaigns for mAbs to more challenging ATMPs for >100 customers. You are in safe hands.
Our service is underpinned by our expert project management team who will keep you informed every step of the way.
Drug development and manufacturing occurs in a dynamic environment. We work flexibly, where possible, to accommodate any changes that you may require.