New Drug Modalities: An Insight into the Future Therapeutic Landscape Opportunities
By David Collins, Commercial Director, Symbiosis
The biopharmaceutical industry is experiencing a paradigm shift in the treatment of complex diseases, particularly oncology, thanks to a surge of innovation and novel drug modalities. While traditional drug platforms have been effective, the industry is now embracing new go-to-market models, personalised treatments and combination therapies to cater to highly stratified patient populations. As the industry continues to grow rapidly, companies must reflect on the changes and challenges ahead, including supply and demand, and the impact of key trends on the industry.
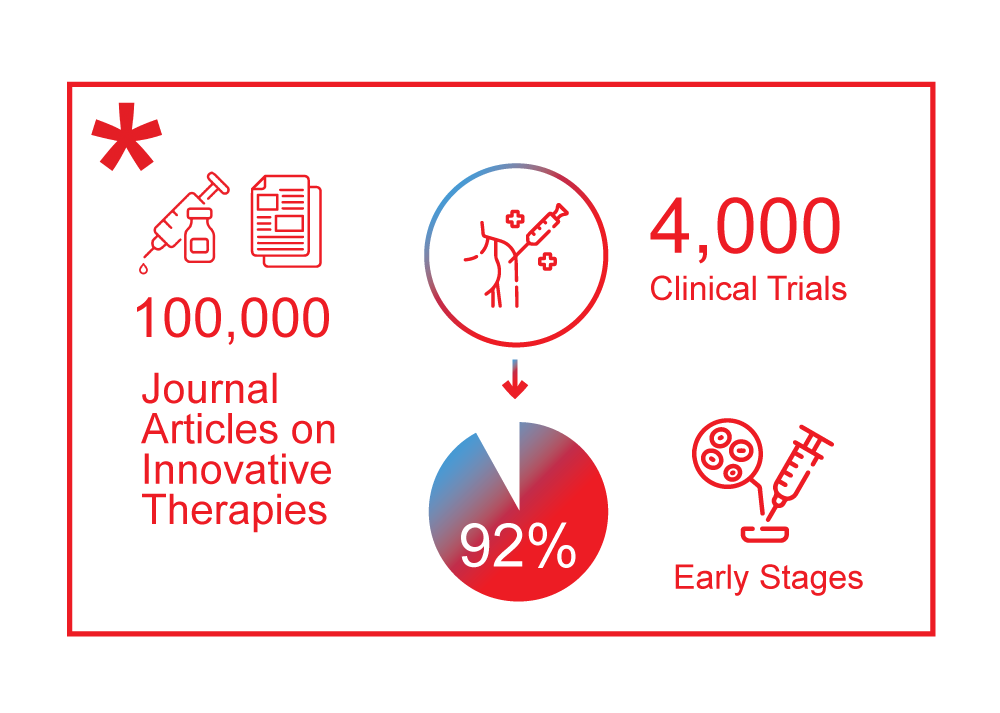
The development of new modalities has reached a turning point after decades of progress. In 2021, around 100,000 journal articles were published on these innovative therapies, and there are currently about 4,000 clinical trials underway, with 92% in the early stages. Over the past two decades, about 1,500 new companies have been established to develop new treatment modalities and technologies, with around 150 going public. The pace of new modality approvals is ever-increasing, with nine new modality drugs approved in the last year alone. These new modalities offer incredible opportunities to revive pharma R&D productivity and change the biological targets landscape.
New drug modalities can be grouped into three categories according to their maturity, as reflected in their technological advancement, clinical application, and commercial readiness. The past 20 years have seen more than 17 new modalities developed, spanning all three categories.
Many new drug types have emerged in the past couple of decades, including gene and cell therapies, RNA drugs, and complex biologics. These modalities have passed initial proof of concept, are progressing through late-stage clinical trials, and being launched commercially. Small-molecule drugs, recombinant proteins, and monoclonal antibodies are the foundation of many existing treatments and have been clinically proven. Therapeutic antibodies have become the predominant class of new drugs developed in recent years, with over 79 therapeutic monoclonal antibodies approved by the US FDA. Antibodies have become the new backbone of the pharmaceutical industry and have been approved for over 30 targets and diseases, most commonly cancer.
However, many potential new modality therapeutics are still in the proof-of-concept stage, being in preclinical development or early clinical trials to demonstrate their initial efficacy and safety. Only a few have gone through pivotal studies or regulatory approvals. For instance, oncolytic viruses, mRNA-based therapeutics and vaccines are still emerging. However, the pace of new modality approvals is picking up, with close to 30 new modality drugs approved in the last five years and about 50 in registrational trials.
Success with new modalities requires not only specific R&D domain knowledge but also experience in regulatory, manufacturing, supply chain management, and commercialisation issues. These modalities are complex and require a diverse set of competencies and knowledge, as each modality has unique manufacturing requirements. Therefore, companies must evaluate carefully the capabilities of their teams and partners to ensure that they can meet the specific requirements of each drug class. The ability to adapt, learn, and act is also crucial in navigating the challenges of new modalities. This horizon expansion across emerging new modality developments is a driving force behind the rise in the requirement for aseptic fill finish contract manufacturers. Being able to partner with a contract manufacturing organisations (CMO) with the capabilities to expedite the manufacturing process due to their capabilities is a significant propellant in the journey to commercialisation.
The emergence of new drug modalities has brought about new challenges in the selection of (CMOs). Emerging Biotech companies must understand the maturity level of the modalities already in the market, along with the many factors that influence decisions about which modalities and diseases to target. The selection of CMOs for manufacturing new modalities requires a diverse set of competencies and knowledge, as each modality has unique manufacturing requirements. For example, the manufacturing of gene therapies requires specialised facilities and expertise in viral vector production, such as we have here at Symbiosis. Therefore, companies must carefully evaluate the capabilities of CMOs to ensure that they can meet the specific manufacturing requirements of each drug type.
In addition, the regulatory requirements for new modalities are complex and evolving, and CMOs must have a deep understanding of the regulatory landscape to ensure compliance. The FDA requires that NDAs provide sufficient information, data, and analyses to permit FDA reviewers to reach several key decisions, including whether the drug is safe and effective for its proposed use(s), whether the drug’s proposed labelling is appropriate, and whether the methods used in manufacturing the drug and the controls used to maintain the drug’s quality are adequate to preserve the drug’s identity, strength, quality, and purity. Therefore, companies must select CMOs that have a proven track record of regulatory compliance and can provide the necessary documentation to support regulatory submissions.
When collaborating with Symbiosis, clients soon understand that they are buying into our quality system. Our value is in our systems, processes and experts that help deliver in a significantly truncated timeline due to our rapid access to manufacturing slots and our ability to leverage our manufacturing process efficiency. And, it is the ability to deliver these outlier characteristics that sustain our reputation as a leader in the clinical or small-scale commercial batch manufacturing in the CMO industry.